Celloxy delivers hypoxic air gas. Hypoxia influences the human body in several ways and causes changes that have an impact in the health. Therefore, the classification of the technology as medical is non-negotiable. Any IHHT therapy should be performed with a device carrying a medical CE. It is our commitment to always comply with all regulatory, quality, and legal requirement in EU.

Celloxy technology is housed in the TUR tower. Its compact construction and its classy design fits to most of therapy environments. The hypoxic gas generator is enclosed in a separate module, making the service of the system extremely simple. Furthermore, the module has a sound insulation that absorbs a big part of the sound frequencies generated by the compressor.

-
Hypoxic Index for each therapy– Definition of therapy intensity
-
Two types of hypoxic test for defining optimal O2% and Hypoxia Resistance type
-
BOLT & HRV Test
-
Change O2 and cycle time during the session
-
Intelligent reoxygenation
-
Analysis of each session & comparison of two therapy sessions

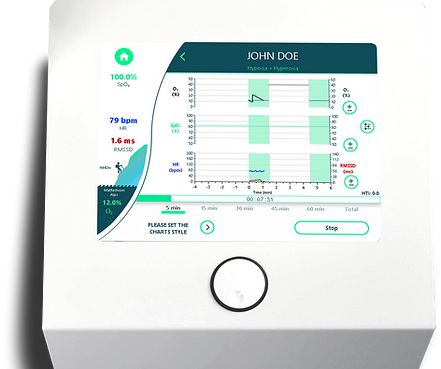
-
Built-in GDPR compliance
-
Enhanced safety by setting two cut offs SpO2 and HR, patient and system
-
Biofeedback and Manual Settings
-
Plotting of HRV and Hypoxic Index over time for progress monitoring
-
Self-Calibration Routine
-
Hyperoxic Preconditioning

SOFTWARE
SPECIAL FEATURES
HRV
Celloxy allows the real time measurements of the RMSSD. After saving the test, the following parameters are calculated and stored. RR & HR | Non – Linear Poincare plot | Graphic & numerical SD1, SD2, SD2/SD1VLF, LF & HF (peak, %, n.u.), LF/HF, mean RR, SDNN; mean HR, sdHR, RMSSD, SDSD, RRvrnc, NN50, pNN50(%).
In addition, there is the possibility to visualise the test as a Periodogram & Poinicare plot.
HYPOXIC TEST 2
Definition of the resistance type. This is an extremely important test, as it warns about possible complications during the therapy. The time that it takes the patient to reach the SpO2 value when supplying hypoxic air mixture is counted. In a second stage the time used to recover and return to the baseline SpO2 is counted. Based on this test, the chance of the patient developing AMS during a therapy is excluded. The result is also used to set the optimal O2 decrease rate during therapy.



Every aspect of the celloxy hardware is designed to be flexible and ensure flawless operation. The storage and memory modules are easily expandable. The device tackles the most demanding professional workloads. It checks and monitors five vital signals hundred times per second for the whole duration of a session. It allows connection of a second monitor. The database stores patient, therapy and protocol information and can be configured to deploy data coming from multiple devices. The hardware reviews the status and ageing of components and notifies the user for preventive actions.



